“Albenmix 10% / 100 mg (albendazole) powder Dewormer 1KG” has been added to your cart. View cart

Spirsol 600 mg for dogs and cats 20 tablets
74.90 € Original price was: 74.90 €.62.23 €Current price is: 62.23 €.

Cefasep 750 for dogs 6 tablets
33.99 € Original price was: 33.99 €.27.79 €Current price is: 27.79 €.
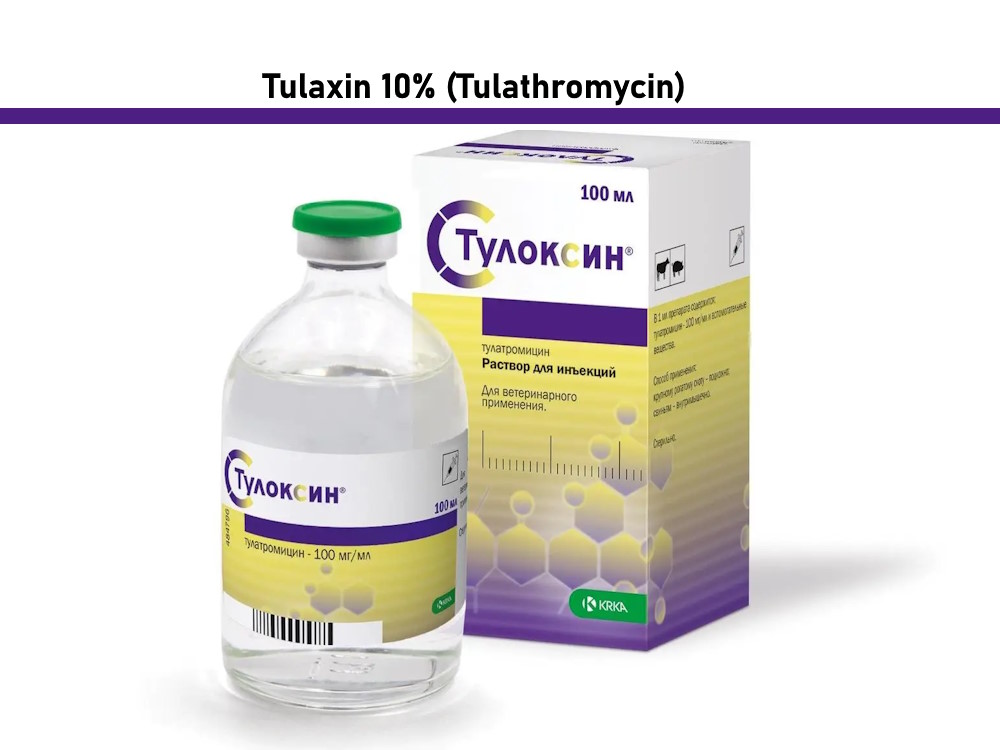
Tulaxin 10% Solution *inj 100ml
350.25 € Original price was: 350.25 €.317.20 €Current price is: 317.20 €.
| Animals | , |
|---|---|
| Brands | |
| Form | , |
SKU:
Tulaxin 10%-INJ*-100ml-KRKA
Category: Antibacterial ♦ Antibiotics
Tags: Cattle, Pigs, Tulathromycin
Description
Tulaxin 10% (Tulathromycin) analog Draxxin, 100 ml
Tulathromycin (Tulathromycin 100 ml)
1 ml of the drug contains 100 mg of the active substance tulathromycin.
Solution for injection is a clear solution from colorless to slightly yellow or slightly brown.
Used to treat bacterial infections of the upper respiratory tract
One injection is a full course of treatment.
Duration of action is up to 15 days.
Maximum concentration in the lungs.
High efficiency during prophylaxis in groups of animals.
Tulathromycin is a semisynthetic bacteriostatic macrolide antibiotic that inhibits protein biosynthesis by selectively binding to bacterial ribosomal RNA. In cattle, tulatromycin is rapidly absorbed and slowly excreted from the body. Maximum blood concentrations are reached within 30 minutes after administration.
Cattle Treatment and metaphylaxis of respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni and Mycoplasma bovis susceptible to tulathromycin. The presence of the disease in the herd should be established before metaphylactic treatment. Treatment of bovine infectious keratoconjunctivitis (BIK) associated with Moraxella bovis susceptible to tulathromycin.
Pigs Treatment and metaphylaxis of respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Mycoplasma hyopneumoniae, Haemophilus parasuis and Bordetella bronchiseptica susceptible to tulathromycin. The presence of the disease in the herd should be established before metaphylactic treatment. The product should only be used if the disease is expected to develop in the udder within 2-3 days.
Sheep, goats Treatment of the initial stages of infectious podognematitis (foot rot) associated with virulent Dichelobacter nodosus, requiring systemic treatment.
Dosage Cattle. Subcutaneous use. Single subcutaneous injection of 2.5 mg tulathromycin/kg body weight (equivalent to 1 ml/40 kg body weight). For treatment of cattle weighing more than 300 kg, divide the dose so that no more than 7.5 ml is administered per site.
Pigs. Intramuscular use . Single intramuscular injection of 2.5 mg tulathromycin/kg body weight (equivalent to 1 ml/40 kg body weight) in the neck.
For treatment of pigs weighing more than 80 kg, divide the dose so that no more than 2 ml is injected into one site.
In the case of any respiratory disease, it is recommended to treat animals early in the course of the disease and to assess the response to treatment within 48 hours after injection. If clinical signs of respiratory disease persist or worsen, or if relapse occurs, treatment should be changed to another antibiotic and continued until clinical signs resolve.
Sheep. Intramuscular use. Single intramuscular injection of 2.5 mg tulathromycin/kg body weight (equivalent to 1 ml/40 kg body weight) in the neck.
To ensure correct dosing, body weight should be determined as accurately as possible to avoid underdosing. The cap can be safely pierced up to 20 times. When treating groups of animals in a single pass, use a withdrawal needle placed in the vial stopper to avoid over-pulling the stopper. The withdrawal needle should be removed after treatment.
Contraindications: Do not use the drug in cases of hypersensitivity to macrolide antibiotics or to any of the excipients.
Do not use simultaneously with other macrolides or lincosamides.
Specific warnings for specific target species:
Sheep : The effectiveness of antimicrobial treatment of foot rot may be reduced by other factors such as wet environmental conditions and poor farm management. Therefore, treatment of foot rot should be combined with other herd management measures, such as dry conditions. Treatment of benign foot rot with antibiotics is not considered appropriate. Tulathromian has shown limited efficacy in sheep with severe clinical signs or chronic foot rot and should therefore only be used in the early stages of foot rot.
Special precautions for use in animals
Use of the product should be based on susceptibility testing of bacteria isolated from the animal. If this is not possible, therapy should be based on local (regional, farm level) epidemiological information on the susceptibility of the target bacteria. Official, national and regional antimicrobial policies should be taken into account when using the product.
Use of the product deviating from the instructions given in the SPC may increase the prevalence of bacteria resistant to tulathromycin and may decrease the effectiveness of treatment with other macrolides due to the possibility of cross-resistance.
Special precautions to be taken by persons administering the veterinary medicinal product to animals
Tulathromycin is irritating to the eyes. In case of accidental eye contact, rinse the eyes immediately with clean water. Tulathromycin may cause sensitization by skin contact. In case of accidental skin contact, wash the skin immediately with soap and water. Wash hands after use. In case of accidental self-injection, seek medical advice immediately and show the package leaflet or the label to the physician.
Use during pregnancy and lactation: Laboratory studies in rats and rabbits have not revealed any signs of teratogenic, fetotoxic or maternotoxic effects!!
Withdrawal period:
Cattle (meat and offal): 22 days
Pigs (meat and offal): 13 days
Sheep (meat and offal): 16 days
Not authorised for use in animals producing milk for human consumption.
Do not use in pregnant animals intended to produce milk for human consumption within 2 months of expected parturition.
The safety of the veterinary medicinal product has not been established during pregnancy and lactation. Use only according to the benefit/risk assessment by the responsible veterinarian.
Overdose: In cattle in doses three, five or ten times the recommended dose.
Important to remember: Subcutaneous administration of tulathromycin to cattle very commonly causes variable pain reactions and local swelling at the injection site, which may persist for up to 30 days. No such reactions have been observed in sheep and goats following intramuscular administration. Pathomorphological reactions at the injection site (including reversible changes of hyperemia, edema, fibrosis, and hemorrhage) are very common for approximately 30 days after injection in cattle and pigs.
Storage precautions
Keep out of reach of children.
No special temperature storage conditions are required.
Store in the original package.
After opening, store below 25°C.
Shelf life after first opening the container: 28 days.
Do not use this veterinary medicinal product after the expiry date which is stated on the carton after EXP.
The expiry date is the last day of that month.
Volume: 100 ml
Manufacturer KRKA, Slovenia
Delivery
We offer FREE SHIPPING WORLDWIDE for all products. Please check out shipping policy.