Oxytetracycline powder 96%
21.78 € – 168.29 €

Enroksan-MAX 10% (enrofloxacin) bottle 100 ml
39.60 € Original price was: 39.60 €.30.91 €Current price is: 30.91 €.
Pharmasin 1000 (Tylosin) 1.1kg Huvepharma
285.20 € Original price was: 285.20 €.249.37 €Current price is: 249.37 €.
| Animals | , |
|---|---|
| Brands | |
| Form |
SKU:
Pharmasin 1000 (Tylosin) 1.1kg Huvepharma
Category: Antibacterial ♦ Antibiotics
Tags: Antibiotics, Pharmasin, pharmazin, tylan, tylosin
Description
Pharmasin (Tylosin) 1000, 1.1kg – Huvepharma Antibiotics
Composition and form of release
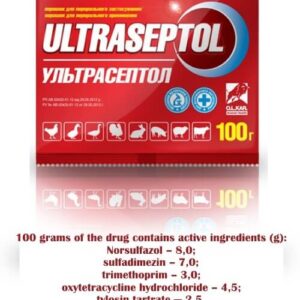
Water-soluble granules for oral administration. Pharmazin 1000 in 1 g of the drug contains 1000 IU of active tylosin, which corresponds to the content of 1100 mg of the antibiotic tylosin tartrate. The medicinal product is obtained by chemical synthesis and does not contain components of animal and plant origin, genetically modified products. Produced packaged in foil sachets of 1100 g. Each package unit is supplied with instructions for use.
Pharmacological properties
The active ingredient of Farmazin 1000 tylosin is an antibiotic from the macrolide group, it is active against most gram-positive and some gram-negative bacteria, including staphylococci, streptococci, corynebacteria, clostridia, pasteurella, erysipelotrix, spirochetes, chlamydia, treponema and chiodizmenteria. The mechanism of action of tylosin is to suppress protein synthesis at the ribosomal level.
When administered orally, the antibiotic is rapidly absorbed into the bloodstream from the gastrointestinal tract and penetrates into almost all organs and tissues of the body. The highest concentration of tylosin is found in the lungs, liver, mammary glands and kidneys. The therapeutic concentration of the antibiotic after a single use of Farmazin 1000 granules remains in the body for 15-24 hours. In addition, due to the peculiarities of the mechanism of action, pharmacin selectively accumulates in tissues with low pH values, that is, in the foci of inflammation, providing a pronounced and targeted effect. Tylosin is excreted from the body mainly with feces, to a lesser extent – with urine and milk, in laying birds – with eggs.
According to the degree of impact on the body, Farmazin® 1000 belongs to moderately hazardous substances (hazard class 3 according to GOST 12.1.007). In recommended doses, it has no embryotoxic, teratogenic and carcinogenic effects.
Indications
It is prescribed for the treatment of poultry and pigs with gastrointestinal and respiratory bacterial infections caused by pathogens sensitive to tylosin.
Doses and method of administration
Pharmazin 1000 is administered orally with drinking water. The antibiotic solution should be kept in the drinkers constantly during the entire period of use of pharmacin. During the treatment period, pigs and poultry should not receive any other source of drinking other than medicated water.
Before use, the recommended dose of pharmacin is dissolved in a small amount of water (water is added to the granulate), and then this solution is diluted with water to the required concentration.
Avoid direct sunlight on the aqueous solution of pharmacin. The antibiotic solution is prepared daily.
With a therapeutic and prophylactic purpose, Farmazin 1000 is prescribed at a dose of 0.5 g per 1 liter of water to broilers for 3 days, to turkeys for 5 days in the following growing periods:
| Species and group of birds | Bird age (days) | ||||
| 1-3 | 1-5 | 28-29 | 56-57 | 140-141 | |
| Breeding bird | + | + | + | + | |
| Industrial herd | + | + | |||
| Turkeys | + | ||||
| Broiler chickens | + | + | |||
With mycoplasmosis, dysentery and gastroenterocolitis of bacterial etiology, pigs are prescribed Farmazin 1000 at a dose of 0.25 g per 1 liter of water for drinking for 3-10 days.
Skipping the use of the next dose of Farmazin should be avoided, because this can lead to a decrease in the therapeutic efficacy of the drug. If you miss one dose, you must enter it as soon as possible. Further, the interval until the next injection of the drug does not change.
Overdose syndromes have not been identified.
In case of allergic reactions, the use of the drug is discontinued and antihistamines or other symptomatic treatment are prescribed.
Side effects
When Farmazin 1000 is prescribed in accordance with the indications and dosage regimen, side reactions and complications are usually not observed.
Allergic reactions to tylosin in pigs are possible, manifested in the form of erythema, itching, mild edema with a slight prolapse of the rectum, respiratory symptoms that quickly disappear after the drug is discontinued. If no noticeable clinical improvement occurs within 2-3 days, it is recommended to retest the sensitivity of microorganisms isolated from sick animals and poultry to tylosin or replace Farmazin 1000 with another antibacterial drug.
Contraindications
Increased individual sensitivity to tylosin. It is forbidden to use pharmaceuticalsin for laying hens and turkeys, whose eggs are intended for human consumption.
Special instructions
Slaughter of pigs and poultry for meat is allowed 5 days after the last use of the drug. The meat of animals that were forcedly killed before the expiration of the established period can be used for feeding fur-bearing animals or for the production of meat and bone meal. Eggs from poultry treated with pharmacin are not eaten due to the risk of sensitization.
Personal prevention measures
When working with Farmazin 1000, it is necessary to observe the general rules of personal hygiene and safety measures provided for when working with veterinary medicinal products.
When working with the drug, it is prohibited to drink, smoke or eat. After work, wash your hands with soap and water. If Farmazin 1000 gets on the skin and mucous membranes, rinse them with plenty of tap water.
Storage conditions
With precaution (list B). In the manufacturer’s sealed packaging, separately from food and feed, in a dry place, protected from direct sunlight and out of reach of children and animals, at a temperature of 5 ° C to 25 ° C. Shelf life is 3 years.
Delivery
We offer FREE SHIPPING WORLDWIDE for all products. Please check out shipping policy.